1.0 OBJECTIVE:
The Objective of the SOP is the Preparation and Standardization of 0.05M EDTA
2.0 SCOPE:
The Procedure is applicable Preparation and Standardization of 0.05M EDTA in company Name.
3.0 RESPONSIBILITY
3.1 QC-Chemist
4.0 ACCOUNTABILITY
4.1 Head – Quality control
4.2 Head – Quality Assurance
5.0 PROCEDURE:
5.1 PREPARATION
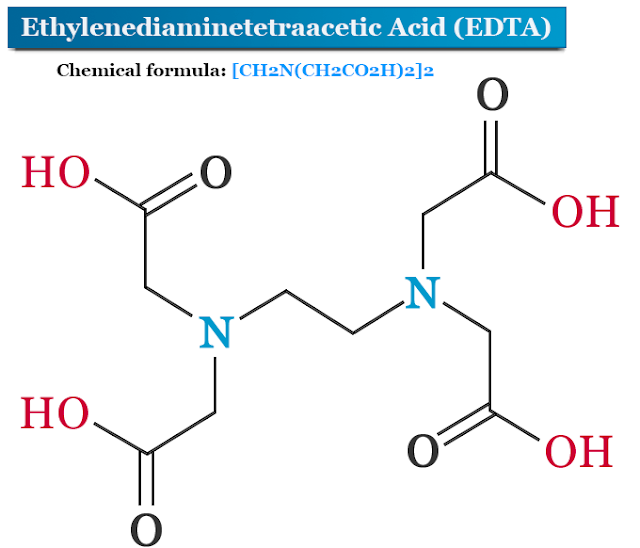
Dissolve 18.6 g of disodium edetate in sufficient water to produce 1000 ml.
5.2 REAGENTS:
5.2.1 Ammonia Buffer pH 10.0: Dissolve 5.4 gm ammonium chloride in 20 ml of water and add 35 ml of 10M ammonia and dilute with water to 100ml.
5.2.2 10M ammonia: Dilute 750mI of strong ammonia solution to 1000 ml with
water.
5.2.3 2 M sodium hydroxide: Dissolve 80 gm of Sodium Hydroxide in 1000 ml of water.
5.3 STANDARDIZATION
5.3.1 Weight accurately about 0.8 g of Granulated Zinc, dissolve by gentle warming in
12ml of diluted Hydrochloric acid and 0.1ml of bromine solution boil to remove
the excess bromine, cool, add sufficient water to produce 200 ml.
5.3.2 Pipette 20 ml of above solution in to a conical flask and nearly neutralize with 2 M sodium hydroxide. Add
about 125 ml water and sufficient ammonia buffer pH 10 to dissolve the precipitate
and add 5 ml in excess.
5.3.3 Add 50 mg of mordant black II mixture and titrate with
the prepared disodium edentate solution until the solution turns to green
point.
5.3.4 Each
ml of 0.1M disodium edetate solution ≡ 0.00654g of Zinc
5.3.5 Perform a duplicate and calculate
the molarity factor (MF) Note down the average value
5.3.6 Calculation
MF =W x 20/200 x 0.00654 x V
Where
W = Weight of Zinc in gram.
V = Volume of EDTA consumed in ml.
MF =W x 20/200 x 0.00654 x V
Where
W = Weight of Zinc in gram.
V = Volume of EDTA consumed in ml.
Note:
1) Discard the solution after 30 days.
2) Restandardise before use
1) Discard the solution after 30 days.
2) Restandardise before use
Store in amber bottle with well
fitted suitable stoppers which prevents access to atmospheric carbon dioxide.
6.0 ANNEXURES
Nil



.JPG)

![Difference between Stability[Shelf life] Specification and Release Specification](https://4.bp.blogspot.com/-O3EpVMWcoKw/WxY6-6I4--I/AAAAAAAAB2s/KzC0FqUQtkMdw7VzT6oOR_8vbZO6EJc-ACK4BGAYYCw/w680/nth.png)






![Cleaning Validation Calculations-Maximum Allowable Carryover[MACO]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiGzQe3wNgJT58HARMOttesykz3va8OngShcTqWy5cOaalJxRSsMcfFzhe1UxZXWTdrCPrcXy5za2onmmI8DSJ2vgkxE7mZQot1dZkAnQ11KCcqCL59rZCtIgWyAKFCo82hiuYZyJ8N6tKIx5WeVkY8nqoB3XVTGLGYb0lSZ4VfQtd8vBJDPWLRYvz2fA/w72-h72-p-k-no-nu/HBEL.jpg)




